Background
Challenge studies using high inoculation levels (typically 10³–10⁵ CFU/g) are commonly performed to quantify log reductions and demonstrate robustness under exaggerated contamination conditions. These tests provide standardized, quantifiable evidence of antimicrobial efficacy and are often required by regulatory agencies such as CFIA, FDA, and USDA-FSIS to validate product performance and establish safety margins.
In parallel, low-inoculum studies, conducted at realistic contamination levels (e.g., ~20 CFU/g), simulate actual post-process scenarios encountered in manufacturing environments. While they do not yield log-reduction data, they confirm practical effectiveness and show whether a treatment can achieve non-detectable levels under real-world conditions.
Together, both approaches provide a complete validation framework: high-inoculum testing for regulatory proof of performance, and low-inoculum testing for applied assurance of food safety in production settings.
Context
Cold-smoked salmon is widely recognized as a high-risk ready-to-eat (RTE) product for Listeria monocytogenes contamination. For products imported into the United States, the FDA enforces a zero-tolerance policy where any detection of L. monocytogenes results in automatic rejection of the shipment.
To ensure compliance with these regulations, an EU-based seafood processor sought an antimicrobial intervention capable of providing residual protection post-processing and throughout frozen export. In this context, Inneo serves as a critical export-enabling tool, providing an added layer of antimicrobial protection that helps ensure compliance with U.S. import requirements and prevents costly shipment rejections or recalls.
Solution
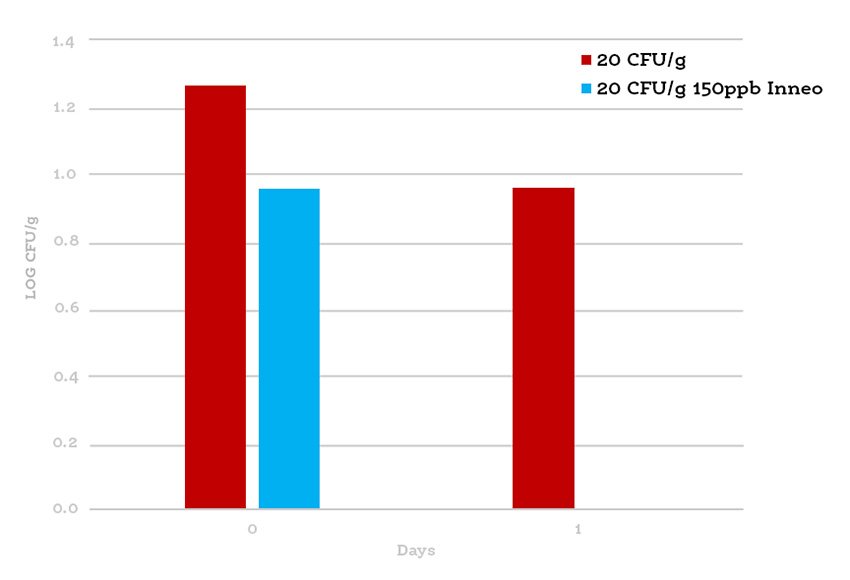
Inneo was applied by spray to the exterior surface of cold-smoked salmon fillets at the standard rate of 150 ppb prior to packaging. Control and treated samples were inoculated with L. monocytogenes at 20 CFU/g to simulate potential post-process contamination and stored under typical refrigerated conditions before enumeration.
Results
Graph 1: Enumeration of Listeria monocytogenes on cold-smoked salmon at 20 CFU/g
Discussion
While traditional high-inoculum challenge studies (10³–10⁵ CFU/g) are designed to quantify log reductions for regulatory proof, this study replicates real-world contamination scenarios typical of controlled seafood facilities. Achieving a non-detectable result from an initial 20 CFU/g inoculum demonstrates Inneo’s practical, field-level efficacy and provides the final layer of protection on the food product that sanitation and testing alone cannot guarantee.
By integrating Inneo at 150 ppb via surface spray, the customer achieved complete elimination of Listeria monocytogenes under realistic processing conditions, enabling compliance with FDA RTE import standards and ensuring product safety, regulatory confidence, and market continuity.
Key Takeaways
Inneo closes the final gap in Listeria control, eliminating contamination from trace levels to absolute zero. And while the FDA enforces the same zero-tolerance standard for all RTE foods, whether produced domestically or imported, U.S. processors often rely on environmental monitoring and preventive controls rather than product-specific interventions. By adding a residual, post-process layer of protection, Inneo strengthens these programs, reduces recall risk, and helps processors consistently meet FDA expectations with greater confidence.



